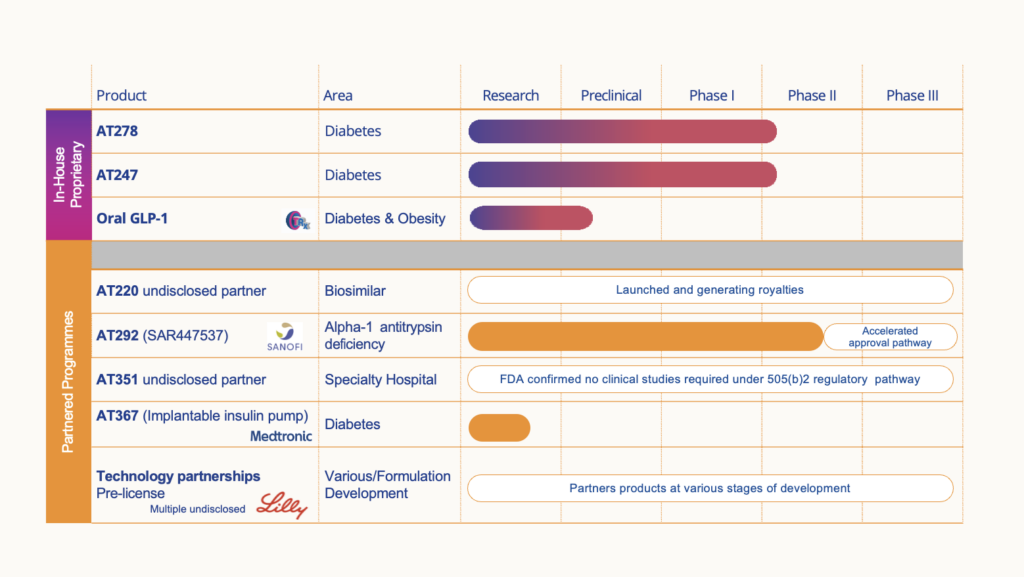
Arecor’s portfolio is significantly de-risked with higher success rates by reformulating existing medicines where the safety and efficacy profiles have already been demonstrated. This enables the use of abbreviated regulatory and development pathways to market thus reducing development risk, lead-times and costs compared with traditional biotech models.
Our two lead products for diabetes, AT278 and AT247, have demonstrated best-in-class profiles when compared in the clinic against the best insulin(s) available to patients today.
Arecor is also leveraging its significant formulation and product development expertise and know-how to develop a novel technology platform for the oral delivery of peptides, with the aim of significantly improving bioavailability and unlocking oral delivery for this important class of therapeutics.
-
Diabetes portfolio
- Two clinical stage next-generation insulin candidates with compelling clinical superiority to best insulins available to patients today
- AT278 is a novel proprietary formulation of an existing insulin, designed to accelerate the absorption of insulin post injection even at very high concentrations (500U/mL). With its best-in-class profile it has the potential to disrupt the market for insulin treatment as the first concentrated, yet very rapid acting insulin for the growing population of people with diabetes with high daily insulin needs as well as to act as a critical enabler in the development of next-generation, miniaturised longer wear automated insulin delivery (AID) systems
- Positive progress is being made towards a strategic partnership with insulin pump manufacturers to further co-develop AT278
- AT247 is a 100U/mL ultra-rapid acting novel formulation of insulin that has been designed to accelerate the absorption of insulin following subcutaneous injection, to enable more effective management of blood glucose levels for people living with diabetes
- The availability of a truly ultra-rapid acting insulin such as AT247 is a critical step towards a fully closed loop artificial pancreas system, a potentially life-changing treatment option for people living with diabetes that has the potential to improve health outcomes and reduce the significant burden of managing this chronic disease
-
Oral delivery of peptides
- Initial efforts focused on the development of an oral GLP-1 receptor agonist (semaglutide) with improved bioavailability when compared to the marketed product, Rybelsus®
- In partnership with TRx Biosciences, combining Arecor’s Arestat™ technology and TRx Biosciences’ novel lipid technology, Lipicore®
- An oral GLP-1 receptor agonist with enhanced bioavailability has the potential to generate significant value in a market that has expanded rapidly given these products’ efficacy in the management of obesity
- Success with Arecor’s GLP-1 receptor agonist programme would validate the application of our technology in the broader and highly valuable field of oral peptide therapeutics across multiple therapeutic areas