SCROLL DOWN
Our Portfolio
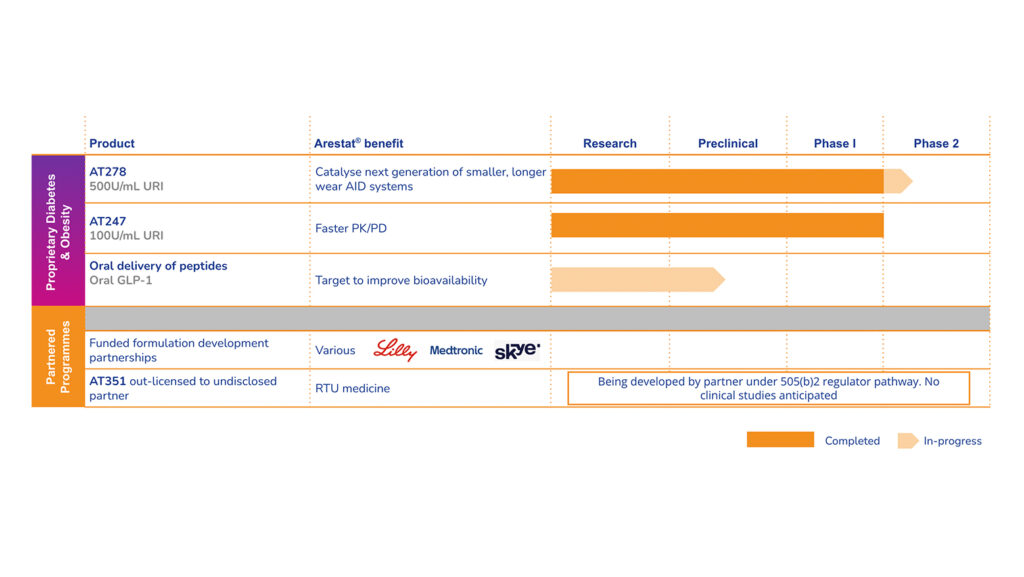
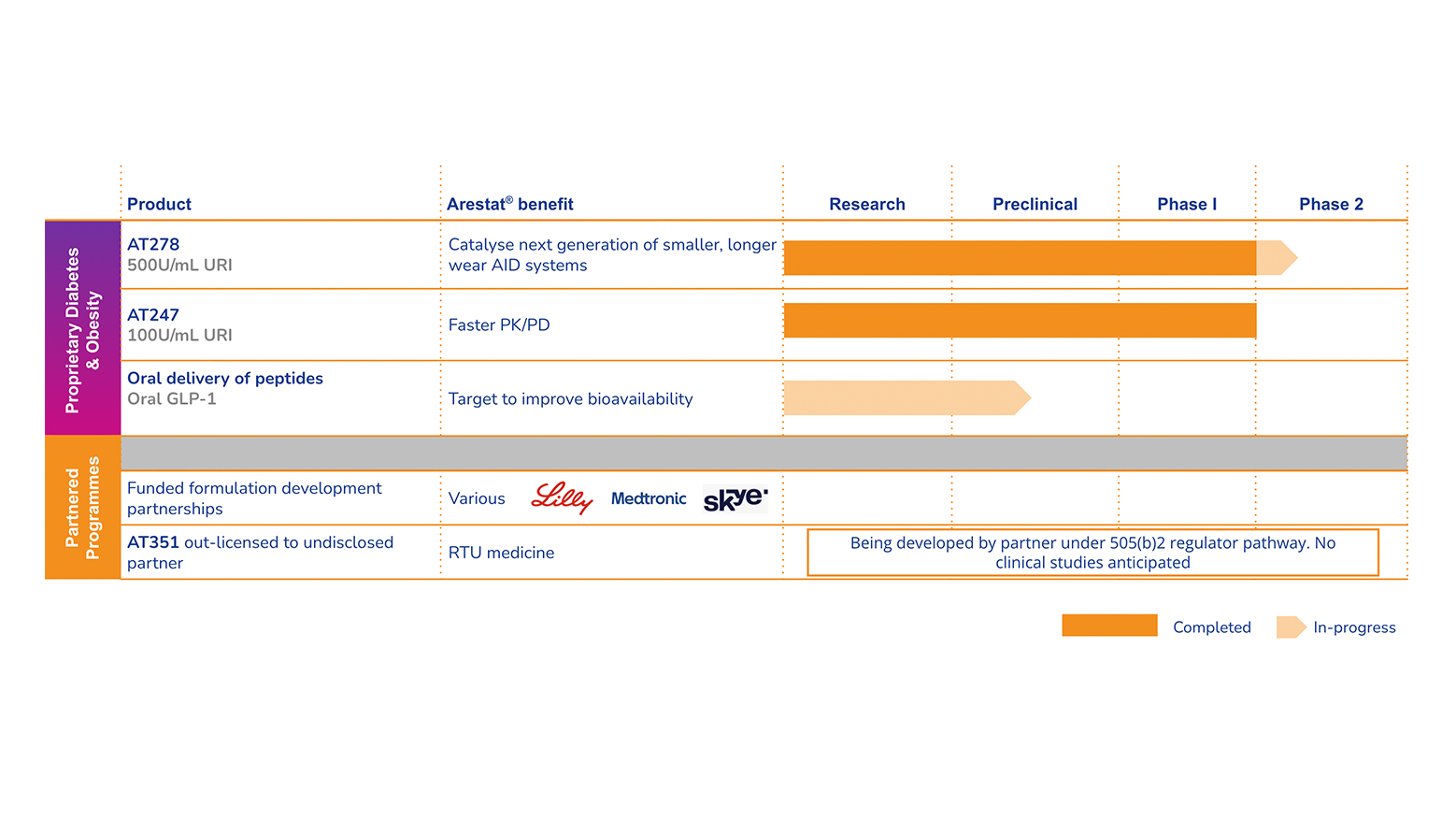
Arecor’s key strength is its ability to develop novel, patent-protected formulations of existing therapeutic medicines that deliver superior product profiles that can bring significant benefits to healthcare providers and patients. In doing so, we build shareholder value. Beyond our partnerships with leading pharmaceutical, biotech and medtech companies, we are progressing a clinical stage proprietary diabetes pipeline of ultra-concentrated and ultra-fast acting insulins. We are also developing a novel technology platform for the oral delivery of peptides.
Arecor’s portfolio is significantly de-risked with higher success rates by reformulating existing medicines where the safety and efficacy profiles have already been demonstrated. This enables the use of abbreviated regulatory and development pathways to market thus reducing development risk, lead-times and costs compared with traditional biotech models.
Our two lead products for diabetes, AT278 and AT247, have demonstrated best-in-class profiles when compared in the clinic against the best insulin(s) available to patients today.
Arecor is also leveraging its significant formulation and product development expertise and know-how to develop a novel technology platform for the oral delivery of peptides, with the aim of significantly improving bioavailability and unlocking oral delivery for this important class of therapeutics.