AT278: The only ultra-concentrated ultra-rapid acting insulin
AT278, a novel proprietary formulation of an existing insulin, has been designed to accelerate the absorption of insulin post injection even at very high concentrations (500U/mL). With its best-in-class profile it has the potential to disrupt the market for insulin treatment as the first concentrated, yet very rapid acting insulin for the growing population of people with diabetes with high daily insulin needs as well as to act as a critical enabler in the development of next-generation, miniaturised longer wear automated insulin delivery (AID) systems.
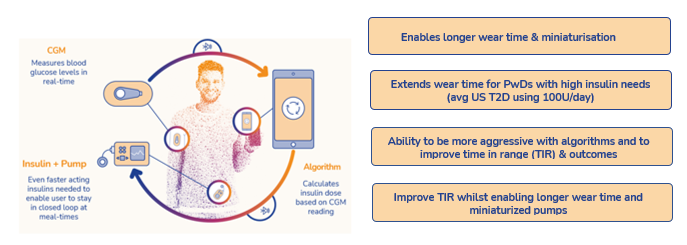
AT278 is the only insulin that has achieved an ultra-concentrated (500U/mL), yet ultra-rapid acting profile. This is a particularly difficult profile to achieve, as when insulin is concentrated it slows time its time action profile, so a slower pharmacokinetic/ pharmacodynamic (PK/PD) profile. Arecor has overcome this with AT278 and has demonstrated clear PK/PD superiority compared with the best insulins available today, in clinical studies with both T1D and T2D patients with a high body mass index (BMI).
- Learn more about Arecor’s Phase I clinical trial of AT278 in Type 2 diabetics with a high body mass index (BMI)
- Learn more about Arecor’s Phase I clinical trial of AT278 in patients with Type 1 diabetes
This places Arecor in an excellent position to bring AT278 to market under a strategic partnership with an insulin pump manufacturer to catalyse the next generation of miniaturized longer wear AID systems to greatly improve outcomes and reduce the burden of care for more people living with diabetes.
AT247: Potential to facilitate a fully closed loop artificial pancreas
AT247, a novel proprietary formulation of an existing insulin, has been designed to accelerate the absorption of insulin post injection. With its best-in-class profile it has the potential to enable a transformational fully closed loop artificial pancreas system where there remains a need for even faster acting, more physiological insulins.
In two Phase I clinical trials in patients with Type 1 diabetes, including a trial to specifically investigate the potential of AT247 when delivered by subcutaneous infusion via an insulin pump over a period of 3 days, this novel insulin formulation has demonstrated faster insulin absorption than currently available, gold standard, rapid acting insulins, reinforcing its potential to enable a fully closed loop artificial pancreas system, a potentially life changing treatment option for people living with diabetes.
Both clinical trial and real-world evidence show that closed loop systems are more effective in keeping blood glucose in a healthy range than standard care, which entails regular measurement of blood glucose level by the patient. The availability of AT247 with its ultra-rapid acting PK/PD profile will be a key component in the move from the currently available systems to those that are fully automatic and require limited input from patients, allowing them to ‘fully switch off’ from worrying about dipping into hypoglycaemia.
- Learn more about Arecor’s Phase I clinical trial of AT247 in Type 1 diabetics when delivered via insulin pump