This website uses cookies so that we can provide you with the best user experience possible. Cookie information is stored in your browser and performs functions such as recognising you when you return to our website and helping our team to understand which sections of the website you find most interesting and useful.
AT247 – Ultra-Rapid Acting Insulin
Our lead product, AT247, is an Arestat™ enabled formulation of insulin designed to accelerate insulin absorption post injection, to enable more effective management of blood glucose levels for people living with diabetes, particularly around difficult to manage mealtimes. It has been designed to achieve pharmacokinetic and pharmacodynamic properties that more closely match the physiological insulin secretion profile of a healthy individual without diabetes, and hence, significantly improve blood glucose control and enable flexibility of insulin dosing as well as the clinical benefits of inhibiting both hypo and hyperglycaemia.
AT247 is under development as a superior ultra-rapid acting insulin for patients with Type I and Type II diabetes who self-administer insulin via multiple daily injection or continuous infusion (pump therapy) and is compatible with all delivery formats.
AT247 demonstrated favourable results with a faster acting and superior glucose lowering PK/PD profile when compared to currently available gold standard rapid acting insulins, Novo Nordisk’s NovoRapid® and Fiasp® in a European Phase I first-in-man study.
Following on from these positive data, in a US-based Phase I clinical trial AT247 delivered significantly accelerated insulin absorption and early exposure (PK profile) compared with NovoLog® and Fiasp®, meeting its co-primary endpoint.
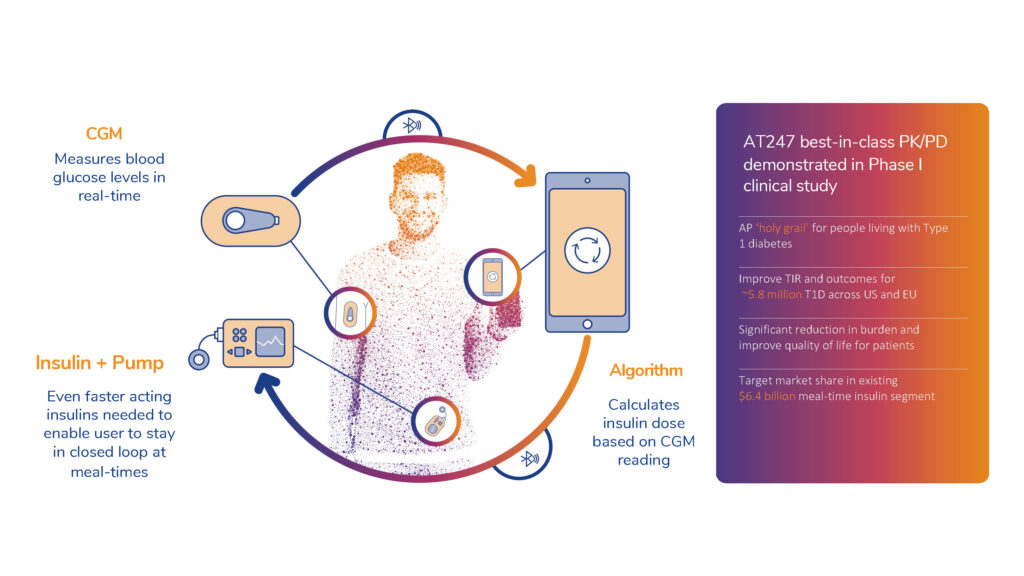
This early clinical evidence suggests that AT247 may also facilitate a fully closed loop artificial pancreas, a potentially life changing treatment option for people living with diabetes.
The Medical Need
There is an urgent need for a more rapid acting insulin analogues to deliver improved patient outcomes. By combining both accelerated absorption and faster onset of action of insulin post injection, the following clinical benefits and improved patient healthcare outcomes are possible:
- Improved post prandial glucose (PPG) control enables the patient to closely manage and control their blood glucose after eating a meal, leading to improved long-term outcomes.
- More Time In Range (TIR) is defined as the % of time spent in the target blood glucose range. More time spent out of range, either too high (hyperglycaemia) or too low (hypoglycaemia) results in long term health complications such as cardiovascular disease, kidney and eye damage. Improving PPG and TIR, particularly around difficult to control mealtimes, has the potential to improve long term outcomes and reduce mortality for people living with diabetes.
- Ultra-rapid acting insulin products allow greater flexibility around the timing of dosing insulin rather than dosing at a specific time before eating. The combined fast absorption and early onset of action of AT247 may enable greater control and flexibility for patients.
- Ideal insulin for pump therapy (continuous insulin infusion). Systemic exposure and clearance of current rapid insulins is not fast enough to support closed loop artificial pancreas (AP) delivery systems without risking significant dose stacking and life threatening hypoglycaemia. AP systems monitor patient glucose levels continuously by a continuous glucose monitor and from these readings the correct insulin dose is automatically administered to the patient via a wearable insulin pump, at any given time, 24 hours a day. ‘Closing the loop’ remains the panacea for insulin delivery and treatment. There is a critical unmet need for even faster acting insulins such as AT247 to enable a true artificial pancreas.
The Market
Diabetes has reached pandemic level worldwide, with 1 in 10 adults living with diabetes there is a heavy health and financial burden on every nation in the world.
- Currently there are ~ 537m adults living with diabetes worldwide, and that number is expected to rise to 643 million by 2030 and 783 million by 2045.
- Within this, there are ~56m daily insulin users, creating a $22bn global insulin market.
Even with the gold standard insulins on the market today, there is still a need for faster acting insulins as diabetes care advances towards enhanced monitoring and tighter glucose controls. This, combined with the rise in innovative delivery devices, allied with technologies where a fast and predictable onset of action is essential, offers Arecor’s novel superior insulin formulations the opportunity to pave the way towards the advancement and miniaturisation of the next generation of insulin delivery devices and change the paradigm of diabetes treatment.