This website uses cookies so that we can provide you with the best user experience possible. Cookie information is stored in your browser and performs functions such as recognising you when you return to our website and helping our team to understand which sections of the website you find most interesting and useful.
Our reformulation technology platform, Arestat™, consists of a series of over ten different patented mechanistically defined families of specific combinations of excipients, which when selected and combined with a therapeutic medicine, will deliver novel formulations with enhanced properties that would otherwise be unachievable.
A key strength at Arecor is our ability to develop novel formulations of existing medicines that deliver superior products that can bring significant benefits to patients and in doing so, build shareholder value.
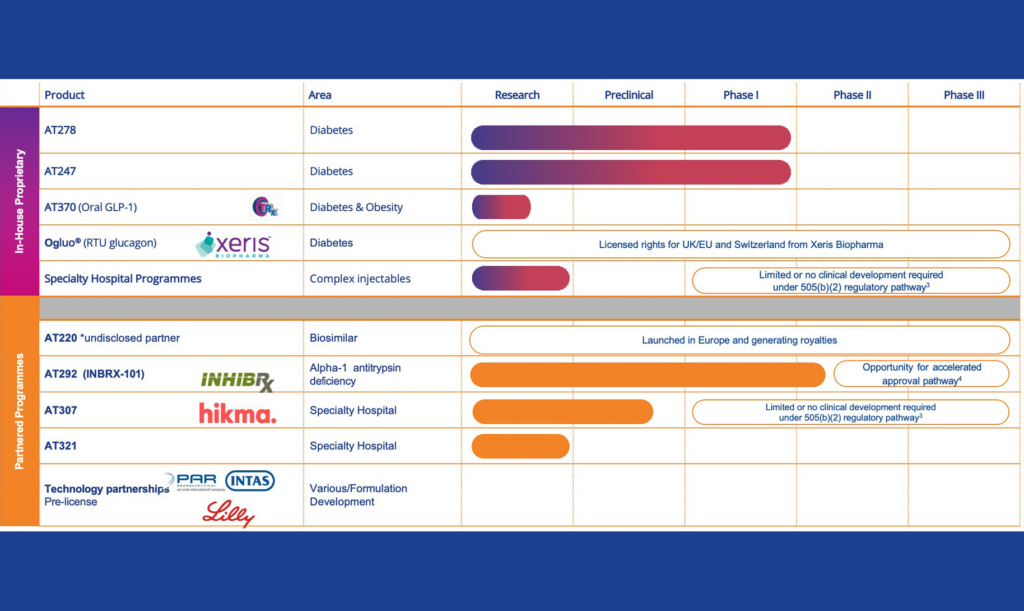
We have an internal pipeline of proprietary products. Within our Diabetes franchise we are developing ultra-fast acting insulins and, in our Specialty Hospital franchise, we are using our technology to develop safer, easier-to-use, injectable products.
Clinical Development Programmes
-
AT247 – Ultra-Rapid Acting Insulin
- Designed to accelerate the absorption of insulin post injection, to enable more effective management of blood glucose levels for people living with diabetes, particularly around difficult to manage mealtimes
- Potential to facilitate an automated insulin delivery via a fully closed loop artificial pancreas, a potentially life changing treatment option for people living with diabetes
- In a first-in-man, European Phase I clinical trial in Type I diabetic patients AT247 demonstrated favourable results with a faster acting and superior glucose lowering pharmacokinetic/ pharmacodynamic (PK/PD) profile when compared to currently available gold standard insulins, NovoRapid® and Fiasp®
- Headline results from Arecor’s latest Phase I clinical trial of AT247 when delivered by continuous subcutaneous infusion via an insulin pump over a period of 3 days, announced in October 2022, showed that AT247 delivered significantly accelerated insulin absorption and early exposure (PK profile) compared with currently available gold standard rapid acting insulins, NovoRapid® and Fiasp®
-
AT278 – Ultra-Concentrated Ultra-Rapid Acting Insulin
Watch our KOL Webinar on the Need for Concentrated and Rapid Acting Insulin
- Designed to accelerate the absorption of insulin post injection, even when delivered at a high concentration, and hence via a lower injection volume
- Potential to enable more effective management of blood glucose levels for the increasing number of people with diabetes with high daily insulin requirements whilst maintaining the convenience and compliance benefits of delivering high insulin doses in a lower injection volume via a single injection
- A truly rapid acting concentrated insulin is a critical step towards the advancement and miniaturisation of the next generation of insulin delivery devices
- Exceeded expectations in a positive Phase I clinical trial, demonstrating a significantly accelerated early PK/ PD profile compared to the same dose of lower concentration, gold standard rapid acting insulin, NovoRapid®
- Headline results for a second Phase I trial in Type 2 diabetic patients, to further explore the product’s potential, are expected 2H 2024